Abstract
Background: Follicular Lymphoma (FL) is the most common indolent lymphoma. While firm data are lacking, global prevalence estimates are >1 million. FL is clinically heterogeneous. Though initial therapy varies, FL responds in most patients, but is not cured. With multiple relapses after variable treatments, each individual patient journey differs. For the 20% of cases relapsing within two yrs (POD24), prognosis is unfavorable, with 5-yr survival ~50%. A stochastic risk of 2-3%/yr for transformation to aggressive lymphoma carries anxiety and poor outcomes when it occurs. Reproducible predictive biomarkers for POD24 or transformation are lacking. FL is also biologically heterogeneous, with varied epigenetic changes cooperating with the t(14;18) translocation dysregulating BCL2.
Given clinical and biological heterogeneity and added complexity of variable treatments, the unmet need of better understanding the underlying biology of FL would benefit from a large comprehensive and integrated clinical and biological database. To garner such new information in FL warrants a new approach to data collection, aggregation, and analysis, needed to generate Real World Evidence (RWE) with longitudinal data collection.
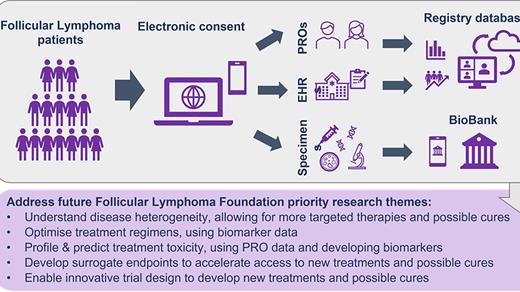
Methods: The Follicular Lymphoma Foundation (FLF) undertook an assessment based on input from multiple stakeholders (patients, clinicians, researchers, life sciences companies), culminating in a workshop to identify key unmet needs in FL and define ways philanthropy could best address these. Key areas: optimizing treatment regimens and sequence; cumulative toxicity profiling and prediction; biomarkers for functional cure; and treatment-centric RWE. The output suggested creating a Precision Medicine Program. This direct-to-patient clinical and biodata registry will be the largest multi-modal registry for patients with FL. Early-stage work in architecting the database models, what data can and will be collected and how it should be stewarded is in progress. We are leveraging experiences of the MMRF (CureCloud®) to accelerate our progress. In addition to clinical and biodata, a biobank for plasma and remnant tissue will be developed. Collecting and aggregating data from varied data sources (Electronic Medical Records (EMR) abstraction, molecular profiling to include genomic, proteomic, and epigenetic data, immune profiling, and patient-reported outcomes) will provide a longitudinal 360-degree view of FL, integrated with outcomes. We continue to seek expert stakeholder input to ensure this comprehensive database will be as broadly useful as possible.
Trial Planning: An IRB-approved clinical study is being designed to permit patients (initially U.S. with later international expansion) to enroll on the FLF website via electronic consent which will allow EMR extraction and collection of any existing tissue. Patients will be asked to supply Patient Reported Outcome (PRO) data serially on validated questionnaires. Existing tissue samples will be sought from all patients for analysis to establish a cumulative molecular database; tissue is not required for enrollment. Specific scientific aims are to use clinical and biologic data to: Optimise treatment care pathways; Profile and predict treatment toxicity; Develop surrogate endpoints for durable response; Enable innovative trial designs. Eligible patients will be at least 18 yrs with diagnosis of FL regardless of stage or prior treatment. A Patient Support Center will assist registrants during and subsequent to enrollment and to maintain engagement. Statistical analysis will mainly be descriptive, based on expected accrual, incorporating multiple imputation techniques for missing data. Data security will be incorporated. Initial pilot of 100 cases from an existing tissue bank with associated EMR data will precede the main direct-to-patient effort.
Discussion: The registry expects > 5000 accruals in the first 6 years. The initial data set of 500-1000 patient cases of FL with molecular and EMR matched data to begin the journey to identify novel biomarkers and molecular targets is expected to be available in 3-5 years. The FLF will report registry construction progress as the program advances. This program fills a unique position of prospectively acquired RWE including biological data as a resource to help identify new biomarkers, drug targets, and facilitate a new era of personalized medicine in FL treatment.
Disclosures
Smith:jannsen: Honoraria; acrotech: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal